Skin Wellness.
Proven by Science.
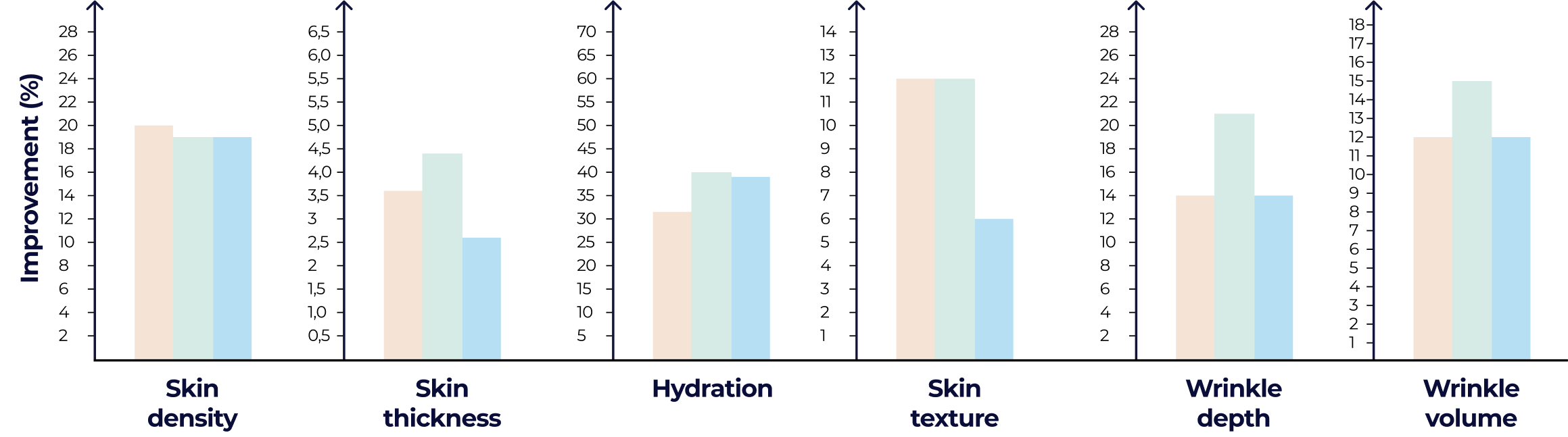
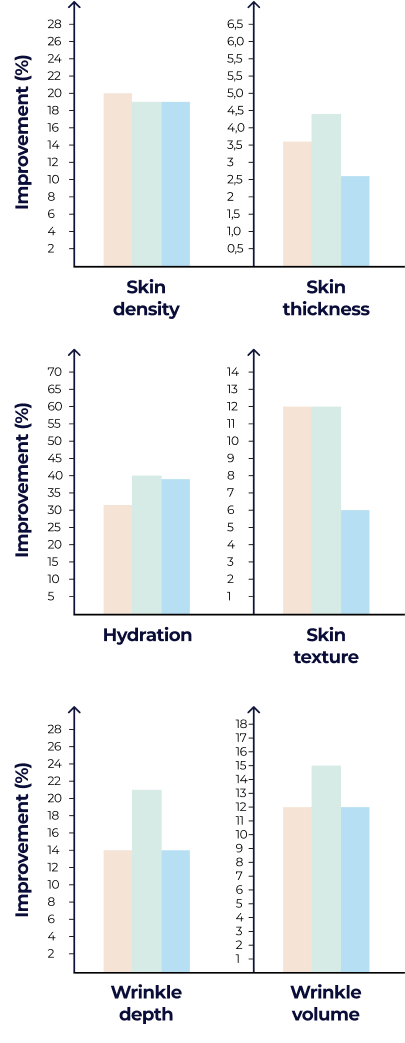
Clinically backed and market-proven. Our best liquid collagen formulations target skin texture, density, and hydration, directly translating into reduced wrinkles and smoother skin.
Collagen
10.000 mg
+ MSM
The highest you can go in terms of skin wellness performance.
Collagen
10.000 mg
.
Collagen
10.000 mg
Highest performance
collagen-only formulation.
Backed by
science
Even after receiving thousands of stellar reviews from end customers of our clients, and seeing visible results of using our products, we wanted clinical proof. So we chose our three best-selling formulations and put them to the test.
The Clinical
Study
The clinical study examined three best-selling Tosla formulations with expert-selected Collagen peptides, produced with Velious™ technology.
An independent institute conducted a randomized, double-blind, placebo-controlled clinical study, involving 107 women aged between 40 to 65 years with a BMI lower than 35.
The primary endpoint of the study was evaluating the capability of test products to promote the synthesis of collagen and elastin fibers in the dermal layer of the skin.
107
Women
12
Weeks
25 ml
Daily intake
Study methods
Dermal density and thickness
Dermis density (a.u.), measured as intensity (Agache and Humbert, 2004), and dermis thickness (μm) was measured using ultrasonography with DermaLab® Series, SkinLab Combo, 20 MHz ultrasound probe (Cortex Technology ApS, Denmark).
A constant gain curve was applied for each volunteer. Skin thickness was measured in μm and intensity as a 0-100 score.
Dermis density (intensity) and thickness measurements were carried out on the right zygomatic area – on the right cheek, in the center between the alar facial groove and the earlobe under zygomatic bone on the outlined measurements area (approx. 4 cm2).
Measurements were repeated two times and the average was calculated.
Texture (topography measurements): Roughness – Ra
Dermis density (a.u.), measured as intensity (Agache and Humbert, 2004), and dermis thickness (μm) was measured using ultrasonography with DermaLab® Series, SkinLab Combo, 20 MHz ultrasound probe (Cortex Technology ApS, Denmark).
A constant gain curve was applied for each volunteer. Skin thickness was measured in μm and intensity as a 0-100 score.
Dermis density (intensity) and thickness measurements were carried out on the right zygomatic area – on the right cheek, in the center between the alar facial groove and the earlobe under zygomatic bone on the outlined measurements area (approx. 4 cm2).
Measurements were repeated two times and the average was calculated.
Wrinkles (topography measurements): Identation index, maximum depth, volumes
Measurements of the wrinkles’ expression were performed by topography measurements using Antera 3D CS (Miravex Ltd, Ireland). Expression of the lateral periorbital wrinkles was assessed with the following parameters: wrinkles indentation index [a.u.], wrinkles maximum depth (mm), volumes – volume of depressions (mm3) at baseline and after 12 weeks of intervention. A medium filter, which is appropriate to analyze fine to moderate wrinkles from 0.5 mm to 2 mm in size was used. Profilometry of the lateral periorbital area was taken on both sides of the outlined measurements area. However, for the quantitative analysis of wrinkles volume and indentation index the side with more expressed wrinkles at baseline and with fewer disturbances such as pigmentations was chosen.
Study design
Study was double-blind, randomized, placebo-controlled four-way study comparing the efficacy of multiple-dose dietary intake of three test products and placebo on skin parameters. It was conducted in Slovenia in Faculty of applied sciences, Institute of cosmetics. Participants tested continuous administration of placebo or one of three investigational products for 12 weeks in order to demonstrate and assess multiple-dose effects.
The study was in full compliance with the principles laid out in the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the VIST – Faculty Applied Sciences (approval ID: 2021/6-ET-SK, date of approval: 12.7.2021) and included in the ClinicalTrials.gov PRS register under record NCT04988412.
The study was performed in compliance with the requirements of local authorities. Before participation in the study, all subjects signed a written informed consent form (ICF).
The study was carried out from October 2021 to February 2022, which was from autumn to wintertime in Slovenia.
Study population
A total of 109 healthy Slovenian female subjects, aged between 40 and 65 years with Fitzpatrick skin phototypes (FT) I-IV were included in the study and divided into four study groups, 27-28 in each.
Each participant was identified by a code that was randomly selected using a computer-generated simple randomization procedure. The codes were sequentially allocated to the participants in the order in which they were enrolled. The study participants, investigators, staff members, and laboratory technicians were blinded to the group assignment. After all the measurements had been completed, the randomization codes were disclosed to the investigators.
Inclusion criteria
Inclusion criteria were: Caucasian female volunteers aged between 40 and 65 years at the time of the signature of Informed consent form (ICF), signed ICF, Fitzpatrick skin phototypes I-IV, signs of skin aging, good general health condition, BMI < 35, willingness to avoid consumption of any food supplements containing methylsulfonylmethane (MSM), antioxidants, collagen, and other protein-based food supplements during the study, willingness to follow all study procedures and keeping a diary during the study (to follow their compliance and palatability), willingness to maintain their living habits and to not begin or change any oestrogen or progesterone therapies, willingness to avoid shaving/depilation of their forearms during the study, willingness not to change cosmetic treatment routine during the study, willingness to avoid rejuvenation treatments during the study.
Exclusion criteria
Exclusion criteria were: pregnancy or breastfeeding, known or suspected allergy to any ingredient of the tested products, changes in dietary habits and dietary supplementation in the last three months prior to inclusion, regular use of food supplements containing MSM, antioxidants, collagen, or other protein-based food supplements in the last three months prior to inclusion, veganism, changes in cosmetic facial and body care routine in the last month prior to inclusion, diagnosed and uncontrolled/untreated/unregulated disease, any clinically significant history of serious metabolic disease, digestive tract disease, liver disease, kidney disease, haematological disease, intake of drugs with any impact on skin reactions (e.g., glucocorticoids, antihistamines, and immunomodulators), any clinically significant acute or chronic skin diseases, skin pigmentation disorders on measurement sites, anticipated sunbathing or solarium visits before or during the study, invasive rejuvenation treatments (e.g. needle rollers, needle mesotherapy, deep/medium-deep chemical peels, laser therapy etc.) in the last 4 months prior to study entry, non-invasive rejuvenation treatments (e.g. radiofrequency, electrotherapy, ultrasound, IPL therapy) in the last month prior to study entry, shaving/depilation of the arms in the last 14 days before inclusion, mental incapacity that precludes adequate understanding or cooperation.
Subjects’ compliance with the inclusion and exclusion criteria was checked before their inclusion in the study. A total of 539 subjects were assessed for eligibility and, 430 of these did not meet the inclusion criteria, 44 subjects declined participation or withdraw due to other reasons before start of the study. Therefore 109 subjects were enrolled onto the study.
Intervention
All subjects consumed 25 mL of syrup daily for 12 weeks with a meal. One study group received investigational product Col-HD (daily dose 25 mL: collagen 10 g, vitamin C: 80 mg), one study group received investigational product ColMSM-LD (daily dose 25 mL: collagen 5 g, MSM: 1,5 g, vitamin C: 80 mg), one study group received investigational product ColMSM-HD (daily dose 25 mL: collagen 10 g, MSM: 1,5 g, , vitamin C: 80 mg) and one study group received placebo product without those active ingredients (25 mL: 0 g collagen, 0 g MSM).
Assesements
Regular checks of the subjects were carried out three times during the study: at the baseline (T0), after 6 weeks (T6), and after 12 weeks of supplementation (T12). To follow their compliance with the protocol, subjects kept a diary of test product intake for the whole 12-week intervention period, and it was checked after 6 and 12 weeks of intervention (T6 and T12) concomitant interview with the subjects).
Dermis density, thickness, skin hydration, TEWL and viscoelasticity measurements were performed at baseline and after 6 and 12 weeks of intervention (T0, T6, T12). Roughness and wrinkles assessments were performed at baseline and after 12 weeks of intervention. The results were obtained between October 2021 and February 2022. All measurements were carried out on subjects lying (or sitting for roughness and wrinkles measurements) in a room with a temperature of 20-25 °C and relative humidity 40-60%. Assessments started after a 30-min acclimatization period in the same atmospheric conditions.
Subjects were instructed to clean their face at least 2 h before the time of measurement and to not apply any cosmetic products on their face 2 h or less before the measurement.
Subjects’ compliance with the inclusion and exclusion criteria was checked before their inclusion in the study. A total of 539 subjects were assessed for eligibility and, 430 of these did not meet the inclusion criteria, 44 subjects declined participation or withdraw due to other reasons before the start of the study. Therefore 109 subjects were enrolled in the study.
Dermal density & thickness
Dermis density (a.u.), measured as intensity (Agache and Humbert, 2004), and dermis thickness (μm) was measured using ultrasonography with DermaLab® Series, SkinLab Combo, 20 MHz ultrasound probe (Cortex Technology ApS, Denmark). A constant gain curve was applied for each volunteer.
Skin thickness was measured in μm and intensity as a 0-100 score. Dermis density (intensity) and thickness measurements were carried out on the right zygomatic area – on the right cheek, in the center between the alar facial groove and the earlobe under zygomatic bone on the outlined measurements area (approx. 4 cm2). Measurements were repeated two times and the average was calculated.
Skin viscoelasticity
Skin viscoelasticity (VE; MPa) was measured using DermaLab® Series, SkinLab Combo, and an elasticity probe (Cortex Technology ApS, Denmark). It was measured on the right zygomatic area on the outlined measurements area (approx. 4 cm2). Measurements were repeated three times and the average was calculated.
Skin hydration
Skin hydration was measured in μS using DermaLab® Series, SkinLab Combo, and hydration flat probe (Cortex Technology ApS, Denmark). The measurement method is conductive and measures the electrical conductivity of the stratum corneum. Measurements were performed on the dorsal forearm on the outlined measurements area (approx. 4 cm2). Measurements were be repeated eight times and the average calculated.
Transepidermal water loss (TEWL)
Texture (topography measurements):
Roughness - RA
Measurement of the facial skin texture was performed by topography measurements using Antera 3D CS (Miravex Ltd, Ireland). The texture of the skin on the left cheek was measured as arithmetical mean roughness – Ra (μm). The texture was measured on the left zygomatic area on the outlined measurements area.
Wrinkles (topography measurements):
Identation index, maximum depth, volumes
Measurements of the wrinkles’ expression were performed by topography measurements using Antera 3D CS (Miravex Ltd, Ireland). Expression of the lateral periorbital wrinkles was assessed with the following parameters: wrinkles indentation index [a.u.], wrinkles maximum depth (mm), volumes – the volume of depressions (mm3) at baseline and after 12 weeks of intervention. A medium filter, which is appropriate to analyze fine to moderate wrinkles from 0.5 mm to 2 mm in size was used.
Profilometry of the lateral periorbital area was taken on both sides of the outlined measurements area.
However, for the quantitative analysis of wrinkles volume and indentation index the side with more expressed wrinkles at baseline and with fewer disturbances such as pigmentations was chosen.
Self assesment of skin parameters & appendages
Self-assessment of skin parameters and skin appendages was performed with Global self-assessment (GSA) questionnaires where subjects evaluated the current state of skin (face, arms), hair, eyebrows, eyelashes, and nails and with Global improvement self-assessment (GISA) where subjects assessed the improvement of skin and skin appendages. GSA and GISA for skin were performed at baseline, after 6 and after 12 weeks of intervention, while GSA and GISA for skin appendages were performed at baseline and after 12 weeks of intervention.
GSA was performed using a 5-grade scale (*-2 – very bad condition, -1 – bad condition, 0 – neither good, neither bad, 1 – good condition, 2 – excellent condition). GISA was performed using 5-grade scale (2” = I completely agree; “1” = I agree; “0” = neither…nor; “−1” = I disagree; “−2” = I completely disagree).
Special conditions for self-assessments – exclusions:
- Subjects that had artificial nails or gel their nails were excluded from the self-assessment of nails.
- Subjects with lash extensions were excluded from the self-assessment of lashes.
- Subjects with hair extensions were excluded from the self-assessment of hair.
- Subjects with permanent eyebrows were excluded from the self-assessment of eyebrows.
All those issues were checked at the screening visit and after 12 weeks.
Profilometry of the lateral periorbital area was taken on both sides of the outlined measurements area.
However, for the quantitative analysis of wrinkles volume and indentation index the side with more expressed wrinkles at baseline and with fewer disturbances such as pigmentations was chosen.